The Essay I Didn’t Want to Finish
This is an essay that I have been promising for some time. I have had to start it from scratch a couple of times, and I am starting it from scratch again now. I tend to get a lot of e-mail about biobutanol, especially after people read the essay that I wrote on the subject last year:
While I wrote about the potential for biobutanol in that essay, I also noted:
I need to spend some time going over the patents and linked reports more closely to see if anything suggests a problem that has been glossed over.
When I first started this essay, I was going to review the literature and outline what I felt needed to happen to make biobutanol a reality. Furthermore, since butanol is about 8% soluble in water, I was going to write that a real holy grail would be to find a microorganism that can tolerate 8% butanol, or one that produces a higher alcohol – one that is completely insoluble in water. That would allow the alcohol that was produced to phase from the water, and eliminate the energy intensive distillation that is typically required. That’s what I was going to write. However, reality intervened.
My Initial Thoughts
Just for completeness, here is what I had originally written:
There has been much press lately about the potential of butanol as an alternative fuel. As I have mentioned before, I spent many years as an engineer in butanol units in Texas and in Germany, and I have received a patent for a butanol process that I invented while working in Germany.
Butanol production via petrochemicals is a very straight-forward process. Once the petro-process was invented, it put the bio-process (ABE) out of business. My guess is that due to the nature of biological processes, the product from the ABE process contains copious amounts of water [I was right]. In contrast, the petrochemical process generally contains product with only 5-10% water. Therefore, the energy requirements for purification are much lower for the petrochemical process.
If butanol could be produced without having to purify it via an energy-intensive distillation, the energy return would be much higher and the costs should be lower. If butanol was completely insoluble in water, for instance, it would float to the top as it was produced and it could just be skimmed off. However, butanol is about 8% soluble in water, which means it won’t start phasing until that concentration is reached. And for a biological process, 8% butanol would most likely poison the microorganisms that were producing it.
However, longer-chain alcohols – pentanol, hexanol, hetpanol, etc. – are essentially insoluble in water. These alcohols would phase out and float to the top as they were produced. Separation would be a snap.
The major problem with butanol is that it doesn’t start to phase out of water until the concentration reaches about 8%. If there was a yeast-based process for butanol that could tolerate concentrations higher than this, you would have the makings of a very cost effective process. Do the fermentation, and then just skim off concentrated butanol. This would be much more energy efficient than a distillation.
What I Learned
While butanol is absolutely a superior fuel to ethanol, the production of butanol from microorganisms is a problem. As I was dreaming about pushing concentration to the point that the butanol starts to phase out, a little research showed the current status quo. Here was the first reality check:
Significant improvements in acetone-butanol (AB) fermentation by Clostridium acetobutylicum must be achieved before it can become an economically viable industrial process (8, 12). Key factors which contribute to the elevated costs of fermentative production of acetone and butanol are the low product titers and low product selectivity. Butanol inhibits cell growth even at relatively low concentrations, and its final titers are limited to ca. 13 g/liter. This and the low product selectivity (i.e., the production of more than one product) result in increased costs for product separation. In addition, continuous cultures have been of limited applicability because solventogenic clostridia degenerate under continuous-culture conditions; that is, they stop producing solvents. (1)
13 grams per liter is about 1.3% butanol. While we can expect improvements in the technology, the yield needs to go up by almost an order of magnitude to keep the distillation energy (and costs) reasonable. It has been demonstrated that you push the conversion to a high level with extreme dilution, but such an approach is simply unworkable from an economical or energy return viewpoint. I won’t address the paper in-depth – I leave that up to others for now – but you can see the dilution approach taken in the paper that is basically responsible for the current biobutanol craze in which 2.5 gallons of butanol per bushel of corn was claimed:
Effects of Butyrate Uptake and Long-term Stability of a Fibrous Bed Bioreactor on Continuous ABE Fermentation by Clostridium acetobutylicum (They have taken this link down; the journal probably complained about them providing the paper for free).
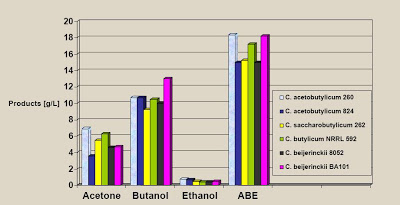
Here is a graphic that shows the extent of the problem (2):
Note that the maximum concentration of butanol achieved was 1.3% for one specific strain. Typical butanol concentrations were just over 1.0%. The total concentration of acetone, butanol, and ethanol amounted to 1.8%. For reference, the petrochemical process produces a product that is somewhere between 85% and 95% butanol. Now you can see why the biochemical process was dropped when the petrochemical process came along. If your objective is merely to produce butanol for some specific application, then the fermentation process can do that – with enormous energy inputs. If your objective is to make fuel with an EROEI of > 1.0, it is nowhere close to being able to achieve that.
Finally, a number of people have written or commented that the work of David Ramey, who runs www.butanol.com, changes all of that. First of all, I certainly don’t want to denigrate Ramey’s work. It is a good contribution. However, it is clear to me that many people do not understand that what Ramey did gets us no closer to cracking the dilution problem. The big hurdle is not a problem of conversion rate or reaction speed. Those are the areas that Ramey addressed, and while those things are nice to have, there is a show-stopper that has not been addressed. That is, if you read Ramey’s patent (which I have done many times), he is still talking about butanol concentrations in the range of 2.5%. That is the problem, not whether the conversion is 25% or 35%.
Butanol is highly toxic to the “bugs”, so it is very difficult to increase the concentration of butanol in the solution. What is needed is a breakthrough that would allow the bugs to thrive at the solubility limit of butanol, which is about 8%. (This would be similar to humans thriving in an atmosphere with 5% oxygen). In that case, excess butanol production would phase out of solution, and separation would be much less energy intensive than a distillation. But you can’t afford to distill off a 2.5% solution of butanol. The energy inputs into the process will be far greater than the energy content of the butanol. I know this from experience. I spent several years as the process engineer in a butanol plant. At a 3% concentration of butanol, we didn’t even attempt to separate it out. Even using relatively cheap (at that time) natural gas, it didn’t make economic sense to extract that butanol. Those levels of butanol were sent to wastewater treatment for disposal.
Believe me, I have a soft spot for butanol. I want to see it work. But right now there are serious issues. That’s not to say that it isn’t worth pursuing. In fact, I am working on it myself. But I have to be realistic.
Higher Alcohols
How about my desire to find microorganisms that could make higher chain alcohols that could be phase-separated?
As a follow-up to earlier studies on the emission of long-chain alcohols from broth cultures of Gram-negative enteric bacteria, E. coli was examined for the production of 1-octanol, 1-decanol, and 1-dodecanol. Ten strains of E. coli cultured in tryptic soy broth were assayed for volatile metabolites using solid-phase microextraction. Long-chain alcohols were produced by all strains with 1-decanol predominating with production ranging from 23.6 ng mL(-1) to 148 ng mL(-1). The production of long-chain alcohols followed the onset of the exponential growth phase of the broth culture. Doubling the concentration of glucose (5 g L(-1)) in the broth had no effect on the concentration of long-chain alcohols produced. (3)
I would have to check on each one to be sure, but my guess is that even though the higher chain alcohols are essentially insoluble, 148 ng/mL (0.0148%) is not going to phase out. (And even if it did phase out, at those low concentrations the reactor would need to be the size of a football stadium to make useful quantities). I was hoping to find that something in the range of 0.5-1.0% could be produced. However, 1% would require the process to become about 70 times as productive. So, it looks like we will colonize Mars before higher chain alcohols will be produced commercially from microorganisms. (Gasification may still have a shot at doing it economically).
Conclusion
Sad to say, but biobutanol is not remotely at the level the hype implies. While research will (and should) continue, the process is currently at least 10 years from any sort of commercial feasibility. And I would point out that “never” falls under the umbrella of “at least 10 years.”
I would note that one “solution” to the poor EROEI problem would be to use heat from coal or nuclear energy to drive the distillation. You may start with 1 BTU and end up with 0.2 BTUs of butanol, and you will emit a lot of CO2, but it may be economically viable.
References
1. Bermejo, Lourdes L., et al.; Expression of Clostridium acetobutylicum ATCC 824 Genes in Escherichia coli for Acetone. Appl Environ Microbiol. 1998 March; 64(3): 1079–1085. Production and Acetate Detoxification.
2. Ladisch, Michael, et al. Research Challenges and Opportunities for Cellulose Conversion Technology in a Dry Mill Pathway; Midwest Consortium for Biobased Products & Bioenergy.
3. Hamilton-Kemp T, et al.; Production of the long-chain alcohols octanol, decanol, and dodecanol by Escherichia coli. Curr Microbiol. 2005 Aug;51(2):82-6. Epub 2005 Jun 27.
Robert – I think your analysis of biobutanol is correct. But can’t we extend this analysis to all fermentative processes? All fermenters 1)have low space time yields, 2)have high capital and operating costs, and 3)give a product that is diluted in water. These limitations are okay if you’re making high value-added products like beverages or pharmaceuticals. But it seems likely to me that fermentation will never be cost effective as a way of producing motor fuels.
Excellent point, tjc!
For the production of high-volume, low-cost products, such as fuels, biological processes can’t compete with chemical processes. Which leads me to believe that the only promosing biofuels would be those produced from low cost feedstock (i.e. waste) using chemical conversion (such as gasification). Hello Congress! Earth calling!
BTW Robert, this is an excellent example of good scientific analysis: objective and honest, to the point where the scientist is troubled by his own conclusion (as what happened with Einstein).
Great example to many who call themselves scientists, but are only willing to follow their subjective instincts.
I’ve got to admit, I’ve been of the opinion that gasification is the way forward ever since I first looked at bio-fuels.
Unless you plonk your ethanol distillery right beside a nuclear facility that would entertain the idea of providing medium grade process steam heat. This would work for to provide for niche applications of high octane motor fuel.
The big problem with gasification is that, once built, the cheapest thing to shovel into it happens to be coal.
I’ve often wondered how much fuel the UK could make if replaced some of our old coal fired power stations with new nukes, and used the existing coal volumes to make diesel. Perhaps on the order of 10% of existing consumption? I’d need to go and look at it…..
Andy
To answer Andy, you need 180 million tons per year of coal to produce 1 million bbl/day of liquids.
I would think biobutanol might be economical with a good source of low temperature waste heat for distillation. Power plant waste heat or geothermal might work.
I would think biobutanol might be economical with a good source of low temperature waste heat for distillation.
Low temperature waste heat won’t work. The water has to go overhead. Considering the amount of water in the mix, you are going to need at least 160# steam. In fact, that’s what we used with 10% water in the mix. These guys are going to have 98% water in the mix.
For reference, streams with 3% butanol and 97% water were sent to the waste disposal ponds at my butanol plant. So even if they get a doubling of the butanol concentration, they are still in waste stream territory.
Cheers, Robert
Robert,
Thanks for the education. Will miss your objectivity and insight. After 25+ years at the biggest of the Sister Oil Companies it is refreshing to see well thought out articles. Have fun with the family.
Thanks
PES
pes – check out the link to the new seven sisters:
The New Seven Sisters
Robert, biofuels that involve fermentation are a dead end. There are two reasons for this, you hit on the first. Yields are low and diluted in water, requiring energy intensive distillation to purify.
The second problem lies in the solution to the first one. Improving yields likely involves genetically modifying organisms to do a better job or finding non-native species to do so. Given the opposition to GM food, can you imagine what opponents would say about super bugs?
UCS: Food and Environment
Look at the controversy over bovine growth hormone. I was recently involved in a project that had a potential impact on fish. The government’s environmental impact said it would affect just 0.1% of the annual fish catch (a mere fraction of the total poplulation). At worst the number was 0.5%, and we demonstrated that we could mitigate 80-90% of the impact. And with mitigation funds could put back more fish than we took out. Yet the project was rejected on environmental grounds. I heard “the precautionary principal” practically everywere I went.
It would be an uphill battle to every get permission to use a GM yeast or organism. I’ve been through these fights before. Can guarantee local environmentalists will have screenings of “The Andromdeda Strain” near your fermentation site.
King,
The problem I see with using GM strains for fuel production is that they can’t compete with wild type. So you have to sterilize your feedstock to make sure you introduce no competition for the GMs. How much is that going to cost? Like tjc said, GM is great for medicine and the like, but not relevant to high volume products.
A counterpoint to 3) on the comment by tjc: fermentation products that are gaseous — such as methane — are still easily separated.
Optimist – I would agree. That is my point. We need a big leap from where we are today to make biofuels competitive. Suppose you could come up with some organism that produce a super cellulase enzyme that happily chews up cellulose and spits out glucose at a very high yield. That organism better be engineered to not last long out in the real world. If it reproduces and competes with other fungi or bacteria, it could have some bad side effects. Even then, there will be some folks that just won’t accept GM organisms no matter how safe they might be.
Can you tell me, what is the solubility of ethanol? My understanding was that ethanol was 100% soluble in water. If that is the case, then isn’t butanol’s 7.7% still an improvement over ethanol, with regards to distillation?
I’m still not comfortable with your conclusion. As of maybe 5 years ago 70% of the cost of corn ethanol was the cost of the corn. The whole processing step only accounted for 30%. In Brazil, ethanol from sugar cane is economic at on oil price of around $35/bbl. So fermentation based fuel production processes can work if you have a cheap feedstock.
The question is should you ferment to ethanol or butanol? I suppose your point is that 8% ethanol is easier to distill than 1% butanol.
Finding the energy to do the distillation is an issue. In Brazil the whole sugar cane plant is cut and trucked into the processing plant. There the sugar is extracted and the plant waste is burned to raise steam for distillation.
US practise is to separate the grain from the plant in the field with a combine harvester. The plant waste is left out in the field. I see no reason why you can’t cut the whole plant and truck it to a processing facility the way the Brazilians do. Then you could burn the plant waste to power your distillation.
I think what it comes down to is that tropical agriculture is more productive that US agriculture. If you want cheap biofuel, you should forget the US and go to the tropics.
If that is the case, then isn’t butanol’s 7.7% still an improvement over ethanol, with regards to distillation?
That was the point. If you can’t get to 7.7% – where the phasing out occurs, butanol is also 100% soluble. And in an ethanol distillation, the ethanol goes overhead. In a butanol distillation, the water goes overhead.
King,
IMHO biofuels means using biomass feedstock to produce (preferably) a liquid fuel. The conversion most likely do not include fermentation, for reasons mentioned above. The obvious biomass to start with would be waste paper. In spite of all the paper recycling you hear about 35 -40% of waste entering US landfills is paper. If I look around my “paperless” office (thanks to Microsoft) I can see why.
The one fermentation process that might work is anaerobic digestion, as Robert McLeod pointed out. A digester can handle pretty much any biomass, provided it includes enough moisture. The resulting biogas would be 50 – 60% methane, and CO2 making up most of the rest, with trace amounts of siloxanes (and other nasties) and hydrogen sulfide (and other stinkies). How hard can it be to clean that up to LNG standard? Also, the Japanese have developed a process, JOGMEC GTL that allows the production of syngas from natural gas without the prior removal of CO2. This could work very well with biogas IMHO.
Am I missing something?
I wouldn’t be too quick to rule out the possibility of breeding a higher-alcohol producing micro-organims. I happen to work in the same building as these guys, who’ve produced a C5-fermenting yeast by old-fashioned selective breeding (GM approaches having various problems as raised by previous commenters).
I forget the exact chemistry, but they found that their yeast produced no ethanol at all (even though they were happily growing on cellulitic breakdown products) until they’d ramped up to a rate-limiting step in the glycolysis pathway, at which point the ethanol-producing enzymes got all the extra action and their yields began to climb.
I know SFA about the biochemistry of large-alcohol production in micro-organisms, but I bet if you started to poke the system surprising things would fall out.
Chris,
You seem to be confusing the substrate (C5 sugars in addition to the normal C6 sugars) with the product (conventional ethanol).
All the comments related to ethanol apply to your neigbors’ process.
This article
http://www.ars.usda.gov/research/publications/publications.htm?seq_no_115=210285 was published yesterday: “These results demonstrate that C. beijerinckii P260 has excellent capacity to convert biomass derived sugars to solvents and can produce over 28 gL**-1 (in one case 41.7 gL**-1 from glucose) ABE from WSH.”
(The paper is accepted for bublication in the Journal of Biotechnology, but is not here http://www.sciencedirect.com/science/journal/01681656 yet)
That is > 2.8 % so I guess that it is possible to find a bug with >8 % butanol produced i.e. a factor of about 3 and that is not unlikey within the next few years.
Mike
Optimist, I was meaning to point out that yields in biological systems are subject to all sorts of peculiar non-linear processes. Selective breeding has taken things from 1% (of whatever trait) to a useful strain many times before. Ever seen a wild brassica? Or a wild silkworm?
That is > 2.8 % so I guess that it is possible to find a bug with >8 % butanol produced i.e. a factor of about 3 and that is not unlikey within the next few years.
A couple of things to note about that. First of all, the 2.8% was not for butanol; it was ABE. The ABE concentration noted in the experiments done to date were around 1.8%. So it is a good bit less than a doubling.
But, they achieved this yield not by the fermentation of wheat straw, but by supplementing the wheat straw with significant quantities of pure glucose. In the experiment, with the wheat straw hydrolysate, they got 25 g/L (2.5%) ABE.
This is an improvement over the current state of the technology, but it will require about 4 times more than that to approach economic removal of the butanol. Therefore, I believe the title – Cost-Effective Bioprocess Technologies for Production of Biofuels from Lignocellulosic Biomass – is seriously misleading.
Still, it is an interesting development. Given the misleading title, though, I will wait for other researcher to confirm the results. Now we only need a 300% improvement to reach the minimum threshhold of economic viability. 🙂 Imagine improving your running speed by 300%, and you start to get an idea of how difficult is the challenge.
Cheers, RR
Apt analysis
What do you think of the method illustrated in Popular Mechanics “Using a complex (and still expensive) photosynthetic process, breakthrough innovators have developed biodiesel and ethanol from an unlikely source that can double its output overnight and just might help give alternative energy the bump it needs: little green goo.”
http://www.popularmechanics.com/science/earth/4213775.html
Algae based biofuels sound pretty cool, and I have written positively on the prospect previously. But one of the key guys on the NREL progect on algal biodiesel pretty much shot down the hype:
The Truth About Algal Biodiesel
Here is a working link back to the PM article.
Pond Powered Biofuels
The inventor might be on to something using cheap manufacturing techniques. I thought algae would work also, but bioreactors would need to be pretty cheap to compete at todays oil prices. I figured $20 per square meter – similar in price to carpeting, and that has to include the plumbing. To replace a small 100,000 barrel per day refinery you would need something like 400 square miles (20 x 20 miles) of bio reactors located close to the refinery. There are some inland refineries in the Texas Panhandle (Borger & McKee) and Oklahoma (Sun Tulsa, Ponca City) that might work. But the big east coast and west coast refineries don’t have access to cheap land required for biofuels.
The present price of n-Butanol is more than $0.94/lb http://www.chemicalonline.com/content/news/article.asp?docid={5592a4df-ce74-4aa2-a294-741ee43150eb} and a new plant for 80,000 metric tons per year of normal-butanol, is scheduled to go into operation during 2008. http://www.chemicalonline.com/content/news/article.asp?docid={68fb086a-4886-4d3f-b3c5-d6d4009c2661}
That is based on technology from Dow that uses propylene and synthesis gas. I guess that they are keeping an eye on the production of biobutanol as well.
If the production cost of biobutanol would be lower than the present method it would have many implications for products and processes that are using n-butanol.
Does anybody have insight in the production cost of n-butanol related to the cost of raw materials (mainly propylene) and energy?
rgsd
Mike qcd_2007
Most butanol is made by first making butyraldehyde, and then hydrogenation to butanol. The butyraldehyde step is done by reacting propylene with syngas in a rhodium and triphenylphosphine (TPP) catalyst mix. The hydrogenation step requires hydrogen, produced from natural gas. Therefore, all of these raw materials are embodied in the final butanol product (as well as the distillation energy and capital equipment).
The single biggest contributor to the price is propylene, and if you plotted butanol price versus propylene price I think you will see them almost always moving together. Off the top of my head, though, I can’t remember what the delta between propylene and butanol tends to be.
Cheers, Robert
The historic propylene-butanol margin is around $0.40-$0.60/lb. Propylene currently sells for around $0.48/lb. Robert is correct in that the butanol market is relatively small compared to polypropylene & propylene glycol – so butanol price tracks its propylene feedstock. Price of propylene depends partly on the raw material costs (crude oil & natural gas liquids) but mostly on the demand for polypropylene – which follows with the global economy.
King,
Interesting article on algal biofuels. I guess we have to wait and see. As long as they are talking about producing biodiesel and ethanol, I have a hard time getting excited, though. Anybody has an idea of how many purification steps it will require to get oil clean enough for biodiesel production from green goo? Same goes for ethanol fermentation: remember the yeast will only grow on glucose (and some other C5 sugars, after tinkering with its genes). It all seems to lead to one conclusion: High energy input + low yield = not good!
I would propose dumping the green goo in an anaerobic digester. The biogas would consist of ~60% methane and mostly carbon dioxide for the rest. Feed the CO2 back to the algae, along with all the nutients in the digested goo and convert the methane to your fuel of choice.
UW-Madison throws their candidate fuel into the mix:
http://www.eurekalert.org/pub_releases/2007-06/uow-ued061807.php
Like butanol, DMF has high energy density and is not soluble in water. The key difference is they claim extracting DMF from water only requires 1/3rd as much energy as distillation.
–doggydogworld
DMF??? WTF? Paint stripper? (I couldn’t resist.) Dimethylformamide would not be on my list of possible fuel replacements. Just a couple of wee technical problems with it:
1) Toxicity – it is believed to cause birth defects and could be a carcinogen
2) Known plastic solvent – DMF is used in the production of plastics and acrylic fibers. DMF won’t play nice with all the plastic and rubber in the average car’s fuel system.
3) Emissions – using DMF as fuel produces NOX as a byproduct of burning the fuel itself (instead of converting elemental nitrogen to NOX from the heat of the engine). This would greatly increase the grams of NOX per mile.
4) Reactivity – DMF is not stable in the presence of either strong acids or bases. It is highly reactive with certain componds, including NaH, which can produce a runaway exothermic reaction.
Other than that, it sounds great. When can I fill up?
The biogas would consist of ~60% methane and mostly carbon dioxide for the rest. Feed the CO2 back to the algae, along with all the nutients in the digested goo and convert the methane to your fuel of choice.
Seperating the CO2 from the CH4 also requires a lot of energy. If you want to convert methane to liquid fuel (C5 & higher alkanes) you give up 40% of the energy just to produce the syngas. Yes, you could make fuel this way but EROI wouldn’t be very good. You are probably just better off to start with coal.
Anybody has an idea of how many purification steps it will require to get oil clean enough for biodiesel production from green goo?
You really just need to get the water out. You could seperate the algae by floculation, then allow the remaining cellular water to evaporate. Probably wouldn’t smell too good. Once you collected relatively dry algae goo, it could go pretty much straight into the refinery process.
Why does even discussing algae and green goo make me think of this:
Green Goo
Were the characters in this movie all peak oil doomers? I’m not too far off here. The title was supposed to suggest a biodiesel feedstock plus the word “lentil”, and according to the story made from plankton. The technical consultant on the film was Frank R. Bowerman, then president of the American Academy for the Environment, who now has a landfill in Orange County, CA named after him.
You really just need to get the water out. You could seperate the algae by floculation, then allow the remaining cellular water to evaporate. Probably wouldn’t smell too good. Once you collected relatively dry algae goo, it could go pretty much straight into the refinery process.
You are right, your Highness, as far as referring to “green” or “clean” diesel. That’s not what they are talking about. Read the fine print.
Biodiesel (FAME) is quite something else. That’s what these guys are after (converting only the lipids in the algal biomass into biodiesel), perhaps in a mistaken belief that biodiesel is cleaner than green diesel, in spite of the fact that biodiesel has many inferior properties from the user’s point of view.
That’s also why they need to make ethanol. Since the lipids is at best ~50% of the biomass, they need to do something with the protein and carbohydrate. They also seem to overestimate the existing capabilities for converting protein and carbohydrate into ethanol, but they’ll find out the hard way, I guess. That’s why I still break skeptical on this one. Once they see things your way…
BTW, is converting (dry) algal biomass into fuel really as easy as “straight into the refinery process”?
If you want to convert methane to liquid fuel (C5 & higher alkanes) you give up 40% of the energy just to produce the syngas. Yes, you could make fuel this way but EROI wouldn’t be very good. You are probably just better off to start with coal.
Nothing personal, your Highness, but you are banking against the Japanese here. I gotta believe that if you get rid of oxygen and go straight CO2 + CH4 -> syngas, efficiency would be more than 40%.
Combine that with systems that can be scaled down without capital costs exploding, and I can see some potential.
Seperating the CO2 from the CH4 also requires a lot of energy.
Not sure I buy that either, especially if you can use some of the CO2 as a reactant for producing syngas. How much easier (and cheaper) than absorption with water and stripping with air can it get?
Whoa – you really are an optimist! The F-T process overall is about 60% efficient, and requires a lot of capital equipment.
It looks like the Japanese are nipping around the edges but haven’t achieved any kind of breakthrough. They may have come up with a better catalyst that allows them to eliminate the air seperation unit to reduce capital costs.
You can’t “recycle” CO2 into more syngas. That requires more energy than you produce from burning the resulting fuel.
But not to worry, I’ve heard that Nancy Pelosi and Harry Reid are trying to change that pesky second law of thermodynamics because it unjustly enriches the evil oil companies.
Whoa – you really are an optimist!
I’ll take that as a compliment.
The F-T process overall is about 60% efficient, and requires a lot of capital equipment. It looks like the Japanese are nipping around the edges but haven’t achieved any kind of breakthrough. They may have come up with a better catalyst that allows them to eliminate the air seperation unit to reduce capital costs.
Hang on there! If F-T requires so much capital, then any savings is good, right? How about simplifying the process? These guys claim to have got rid of the oxygen plant, CO2 removal and syngas conditioning. They also claim some breakthroughs with catalysts.
You can’t “recycle” CO2 into more syngas. That requires more energy than you produce from burning the resulting fuel.
They add steam for that. Cheaper than burning pure oxygen, I imagine.
But not to worry, I’ve heard that Nancy Pelosi and Harry Reid are trying to change that pesky second law of thermodynamics because it unjustly enriches the evil oil companies.
Ah, yes, comic relief out of Washinton DC. Watching the dumbed down media interview these people is becoming like a real life version of “Dumb and Dumberer”.
You’re pretty optimistic yourself, when it suits. Such as your plan to put algal biomass “straight into the refinery process”…
I was wondering does your analysis of butanol covers the work that Dave Ramey is doing at ButylFuel?
Hello Robert and all other posters,
I have been very busy at work, so haven’t had tome to post, just reading all the fascinating feedback to Robert’s excellent analysis. That of course created even more interesting lines of thought! 🙂
A few questions for anyone with information:
1. Given what we are discussing, has anyone heard how the BP/DuPont Chemical biobutanol project is coming? They intended to set up production in the UK using sugar beets as the feedstock. Is this still on track, and what would be the liklihood of success, given what we know?
2.There was some discussion in the string of replies regarding methane to liquid fuel.
We know that methanol can be made from methane at considerable cost. Does anyone know of research being done with hydrogen to create a synthetic liquid fuel from hydrogen/carbon combination to create a synthetic methanol or synthetic propane?
I am becoming more and more convinced that the distilling/biofuels route simply contains too many conversions, and that solar extraction of hydrogen holds more promise. I know, hydrogen is an energy carrier, not an energy source, the conversion efficiency is still too low to be financially acceptable, etc., etc.
But the conversion of sun to hydrogen is beginning to look at least as efficient as the biofuels (and without complicating factors such as consumption of topsoil and fertilizer, freshwater contamination, deforestation, etc), and look what is being spent on that industry, so that it can be passed off as viable.
Anyway, if we are having trouble finding something that works, we seem to be having great success in ruling out the many options that don’t!
🙂
Roger Conner Jr
Robert
First of all, I’d like to congratulate you on your site, I find it quite impressive.
Second, I’d like to know if you have some estimates of butanol’s EROI when produced from fossil sources -in particular from coal and natural gas- as well as the production costs (capital costs + operation costs).
If fossil-source butanol is cost competitive, it could motivate oil companies to start producing it, thus creating the bases of a butanol economy (logistics, standards, engines, etc) which would lower the economic burden on biobutanol. Since this process would take several years, it would fit quite well with the biobutanol R&D timetable to come up with a cheaper alternative.
Cheers,
A
Second, I’d like to know if you have some estimates of butanol’s EROI when produced from fossil sources -in particular from coal and natural gas- as well as the production costs (capital costs + operation costs).
The production cost is highly dependent upon the cost of propylene. Total cost, probably around the cost of propylene plus around $0.30 per pound.
The EROI would be hard to get at. Both the hydroformylation and hydrogenation reactions are exothermic, and the purification step takes about 2 pounds of steam per pound of purified butanol. But then you would have to add in the EROI from the propylene production step. You could get at all of that, but it would be a chore.
But you would probably be better off – in fact I know you would be better off – just burning the natural gas and propylene used to make the butanol, rather than going through all the hoops to make the butanol.
Cheers, Robert
Well, 0.3 $/pound is 666 $/ton (aka Satan’s number, coincidence?). Assuming a low heating value for Butanol of 32 MJ/kg, the production cost is 22 $/MBtu without the cost of propylene. Given that crude oil sells for approximately 10-12 $/MBtu, this is clearly too expensive.
Isn’t there a cheaper way to produce Butanol at much higher scales from syngas?
Wondering if anyone has an opinion on this person’s biobutanol efforts with algae:
http://www.biodieselnow.com/forums/thread/131279.aspx
His website has further descriptions of his process.
The link to “effects of butyaret uptake and long term stability…” unfortunately doesn’t work.
I would be interested in reading that paper, can you post the correct link?
I would be interested in reading that paper, can you post the correct link?
The link was correct; they have just taken the paper offline. The journal probably complained that they were offering the paper for free. Here are some links that they say they do have available:
http://www.butanol.com/page9.html
Cheers, RR
1. The industrial AB fermentation has used hydrogen generated by the fermentation for process heat for distillation, and so didn’t require external (eg fossil) energy inputs for distillative separation.
2. Product titer affects costs, but yield and productivity matter also, and may be easier to engineer. Even considering titer alone, I’d expect substantial benefits to increased titer, with no big break at 8%. Even imagining a two-phase regime, you still need to get rid of the water from the butanol rich phase.
3. Economic comparisons need to be clear about what they are comparing. Is it biomass butanol vs. fossil butanol as a butanol source? Biobutanol vs. bioethanol as a fuel? Biobutanol vs. fossil gasoline as a fuel? In every case, one needs to consider not just plant costs, but also expected future feedstock costs, and byproduct credits (or debits, in the case of net GHG emissions).
Recent economic analyses have been published by Gapes and Qureshi. BP-DuPont have not published their analysis, they are merely building a plant.
The industrial AB fermentation has used hydrogen generated by the fermentation for process heat for distillation, and so didn’t require external (eg fossil) energy inputs for distillative separation.
That is false. Where did you get that information? To purify 1 gallon of butanol, you have to remove 50-100 times as much water. The hydrogen byproduct (which is also mixed with CO2) could only remove a tiny fraction of that. Coal was the fuel of choice for the distillation.
See: Acetone-Butanol Fermentation Revisited by DAVID T. JONES AND DAVID R. WOODS. They discuss the history, and also talk about the high energy cost of distillation. See page 6 for a description of the process, and the role of coal. The paper is publicly available on PubMed for no charge.
In my search for infrmation on DMF (dimethylfuran) as fuel I found the comment by KingofKaty dated 20 June:
KingofKaty said…
DMF??? WTF? Paint stripper? (I couldn’t resist.) Dimethylformamide would not be on my list of possible fuel replacements. Just a couple of wee technical problems with it:
1) Toxicity – it is believed to cause birth defects and could be a carcinogen
etc….
I understand why he would notwant dimethylformamide, but the Wisconsin technology yields DMF dimethylfran.
Obviously he is referring to a wrong chemical.
He is referring to dimethylformamide, not dimethylfuran.
Can anyone comment on phase behavior below 0 degrees C? Would the water freeze and leave a liquid butanol on the surface? The ice accumulation could be used to pre-cool the stream.
Also, any possibility of synthesizing butanol from the much-easier-to-distill ethanol?
See http://www.gevo.com
“still hopeful” asked if one can make butanol from ethanol. The short answer is “yes” – via the process to make what are called Guebert alcohols (combining 2 moles of a shorter alcohol into one mole of a longer chain alcohol with twice the carbons). The longer answer is that it may not make sense to add more cost and energy to ethanol – but I have not done the math on it, nor have I heard anyone working actively on this as a viable route.
Um there are a lot more ways of separating alcohols from water than energy intensive distilation. The easiest way is to add a substance that causes the solubility of butanol to decrease below 1%. If you added one of the higher insoluble alcohols i think the butanol would preferentially disolve in the insoluble alcohol phase, renoving it from the aqueous phase. Other substances that might work would be castor oil. Some crystaline solids might attract butanol out of the solution to form a separate phase. Sulphur night work.
Robert,
I haven’t had a chance to read all comments as yet so this might be a repetition. This development looks promising.
>http://newenergyandfuel.com/http:/newenergyandfuel/com/2008/01/04/butanol-breakthrough-from-e-coli/
How about physical separation of product via osmotic membranes or other non chemical means.
Also Geothermal heat could provide reaction/separation energy instead of Nuclear.
Utilizing food stocks for fuel production seems foolish .
The southern waterways are being choked by Water hyacynth and Kudzu is overunning the land.
Looks like a great source of available biomass to me.
Hi, you mentioned that distilling butanol while it is miscible in water (<7.7%) is energy intensive.
Just a thought; would it be reasonable to distill only up to the point where the butanol concentration is high enough for it to become immiscible (ie evaporate ~3/4 of the water, i’m guessing), then separate by phase separation?
I was thinking that this sort of distillation would require fewer stages and less reflux/reboiling, so would be less energy intensive??
I’d like to correct several misconceptions in the discussion.
Genetically modified microorganisms are one way to make butanol more economic. Genetically engineered microorganisms or GEMs have been used industrially for 30 years for the production of drugs, enzymes and industrial chemicals (e.g. DuPont-Tate&Lyle’s 1,3-propanediol). Such GEMs are fully contained and the microorganism is killed in the process. The EPA regulates GEMs and the process is straightforward although it requires a lot of documentation. GEMs will be used to produce only butanol in highly butanol tolerant microorganisms. DuPont and UCLA’s Jim Liao have publications detailing how only butanol can be produced by GEMs. I don’t believe that E. coli is the proper microorganism but there are many others that can be engineered.
I’d also recommend studying Marlett and Datta and other butanol references which cover the recovery of acetone, butanol and ethanol from water. The acetone, butanol and ethanol along with some water (but not a significant quantity) are distilled from the fermentation broth (traditional clostridia ABE). This is not consistent with the earlier discussion in which its stated the water must all be boiled off. In fact the references show the butanol is ‘boiled’ off.
Good luck to all in pursuit of sustainable sources of energy!
The acetone, butanol and ethanol along with some water (but not a significant quantity) are distilled from the fermentation broth (traditional clostridia ABE). This is not consistent with the earlier discussion in which its stated the water must all be boiled off. In fact the references show the butanol is ‘boiled’ off.
The boiling point of butanol is 117 C. The boiling point of water is 100 C. I have worked in a butanol plant for several years, and distillation was my speciality. Believe me, the water boils off first.
I agree with you that genetically modified organisms are the way to go, but there is a long distance to cover before it is economical to make butanol in this manner.
RR
My question concerning Butanol is what is the true and simple Yields I plan to used Sugar just like you buy at the store I have a source for it at $0.06 per lb. it’s not market price I have a family member in the Sugar business, also we plan to do first stage distillation using a process similar to a woodstove but does have a way to control the heat with in 3 degrees, and we are doing research into Solar Distilling as well but we need the yields using Sugar and can’t find it on the net, and to be honest I do not care if it cost more than gas I have grown do despise the oil giants so bad I just refuse to give them any more of my money.
I remember reading a description of a low energy butanol separation facility. It used a non-water soluble phase that the butanol migrated into and from which it was distilled.
Also, there is a zeolite molecular sieve that can convert methane to gasoline without FT. Patented by Mobil oil decades ago and used in New Zealand in the 1980s?
Many imaginative ways exist to separate chemical cats from skins…
what about using the water used as coolant for nuclear power plants? cant this heated water be used to distill the butanol solution? i heard two thirds of the capacity of nuclear power plant goes as waste heat.
Can’t you just Gas strip the butanol as it is being produced? This is what David Ramey claims he does. What you get when the butanol is condensed back out doesn’t have that much water at all.
Straight distillation isn’t the only way to separate butanol from water and just because it is too expensive to do it that way doesn’t mean there aren’t other cheaper ways.
Can’t you just Gas strip the butanol as it is being produced?
Water has a lower boiling point. You can’t strip butanol from water.
You might be able to solvent extract it, but then you still have the problem of separating the low concentration butanol from the solvent. There just isn’t a way to purify a very low concentration butanol stream that doesn’t require a lot of energy.
And it isn’t that a lot of people haven’t looked at it. Butanol is an important commodity chemical, and every producer has low concentration butanol waste streams to deal with. If there was a cheap way to get it out, it wouldn’t end up in the wastewater treatment ponds.
RR
Water has a lower boiling point. You can’t strip butanol from water.
It worked when I tried it and these people all talk about it working:
http://arsserv0.tamu.edu/research/publications/publications.htm?SEQ_NO_115=148912
http://cat.inist.fr/?aModele=afficheN&cpsidt=4648062
http://www.ars.usda.gov/research/publications/Publications.htm?seq_no_115=170133
I can't find the link on butanol.com any more but at one point david ramey had a paper that talked about his experiments with it and gas stripping worked for him.
And it isn’t that a lot of people haven’t looked at it.
Right don’t you think BP and DuPont have figured out some way to do it economically?
It worked when I tried it
When did you try it? Tell me about your set up and how you recovered the butanol.
Acetone and ethanol will strip out. Butanol? If butanol is coming out with the stripping gas, so is water. But two of the reports you linked to were all ongoing research projects, and there didn’t appear to be an actual report describing quantitative results. The 3rd was from 1993. If the technology was actually effective, every butanol producer in the world would have switched over in the past 15 years.
Right don’t you think BP and DuPont have figured out some way to do it economically?
No I don’t. Here is a short list of companies who have been doing research on butanol distillation over the past 50 years:
BASF, Dow Chemical, Eastman Chemical, Celanese, and Shell. They have all of the financial incentive in the world to invent a cheaper separation technique. So no, I don’t think BP and Dupont – with little butanol experience – suddenly invented a new technology that has eluded all of these other butanol producers over many years.
Readers of this post might be interested in the following article:
Thaddeus Chukwuemeka Ezeji, Nasib Qureshi and Hans Peter Blaschek, “Bioproduction of butanol from biomass: from genes to bioreactors”, Current Opinion in Biotechnology, Volume 18, Issue 3, June 2007, Pages 220-227.
The authors hedge their opinions but largely echo Robert’s conclusion:
“Several recent advances have been made including the development of microbial cultures, process technologies, and use of waste substrates; however, these advances will need to be further developed to run a fermentation-based biobutanol industry that can compete effectively with petrochemically derived butanol.”
Hi Robert,
china is doing a lot of biomass into butanol also. there should be lready 5 bio butanol factories in operation. do you have clues what kind butanol/water separation technik chninese is applying? many thanks